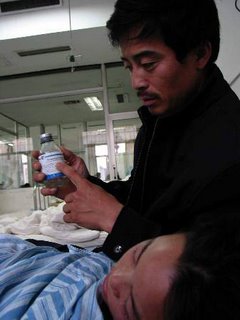

Top: A victim of Xinfu injecta
Bottom: Mr. Qiu Zuyi
Mr. Qiu Zuyi, former manager of a state-owned medicine company, was found dead at his home in Fuyang, Anhui, on November 1. He apparently killed himself, according to Chinese media.
Beijing’s Mirror newspaper said he lived alone in Fuyang, while his wife and son lived in Shanghai. He also left a note with words like “very, very sad,” “no way to let out the pressure,” and “can only die to apologize,” according to Guangzhou-based 21st Century Economy.
The fatal nightmare of Mr. Qiu and his company, Anhui Huayuan Medicine Company, started on August 3, when China’s health ministry issued an emergent notice to all health providers across China, requesting immediate suspension of the use of Xinfu glucose injecta manufactured by the company. The injection fluid was reported to have caused at least dozens of deaths, and other harmful symptom such as sallergic shock, liver and kidney damage, and palpitation.
More than 2.7 million bottles of Xinfu liquid were soon recalled. In mid-October, China’s food and medicine administration announced that the problematic injecta was not sterilized to standard level. During its production process, the factory lowered the sterilization temperature, shortened the time, and overloaded the disinfection container, and therefore impaired the effect of the sterilization. Mr. Qiu was since removed from his post.
Meanwhile, more and more victims and their families around the country started to ask the company for compensation, and family member of some diseased victims came and burn funeral wretch inside the factory.
Ironically, on Friday, the company’s website still boasts its strong emphasis on product quality.
Mr. Qiu used to be seen as the hero of revitalizing a moribund state-owned factory. He applied and was chosen to be the manager in 2000 and had since turned the dying company into profit, and provided job to nearly 2000 people. The factory has resumed its production.
Fake and poor-quality medicine has been a huge problem in China in recent years as some manufactures pursuit profits over quality. The government has become more responsive to such cases, because some of the poor quality medicines kill people and the public is likely to be agitated if they don’t think their call for justice is sufficiently answered.
The dealing with the Xinfu incident appeared to be fairly prompt. Not only executives in the factory, but also local government officials overseeing medicine manufacture received punishment ranging from removal to disciplinary sanctions. The death of the former manager, however, may not put an end to people’s furies and compensation battles.
http://news.sina.com.cn/c/h/2006-10-16/121211250317.shtml
http://news.sina.com.cn/c/2006-08-03/23159650747s.shtml
http://news.sina.com.cn/c/2006-08-03/20039650494s.shtml
http://news.sina.com.cn/c/2006-11-03/234310408073s.shtml
----by Josie Liu
No comments:
Post a Comment